
Adsorption and desorption processes occur everywhere. They play an important role in areas such as surface science, biomaterials, cell and molecular biology, and pharmaceutical development and production, where molecules and nanoparticles interact with various surfaces in different contexts.
Adsorption can be defined as the ‘adhesion’ of molecules from a liquid or gas phase onto a surface. Desorption is the reverse phenomenon, when adsorbed molecules are removed from a surface. QCM-D technology, which is essentially a balance for small masses, can monitor molecular adsorption and desorption processes in real-time by detecting the mass changes following the molecular uptake or release from the surface studied.
Depending on the application and objective of the study, it may be relevant to either understand, characterize or optimize the adsorption or desorption events. Either way, it will be relevant to monitor the amount of material that is being added to or leaving the surface, and it may also be relevant to investigate the rate at which the process occurs. Each time material is added to or removed from a surface, there is a corresponding change in mass, which will be detected by QCM-D in real-time.
As an example, let’s have a look at protein adsorption on two different surfaces, one glass surface and one plastic. As outlined in Figure 1, we follow the steps below.
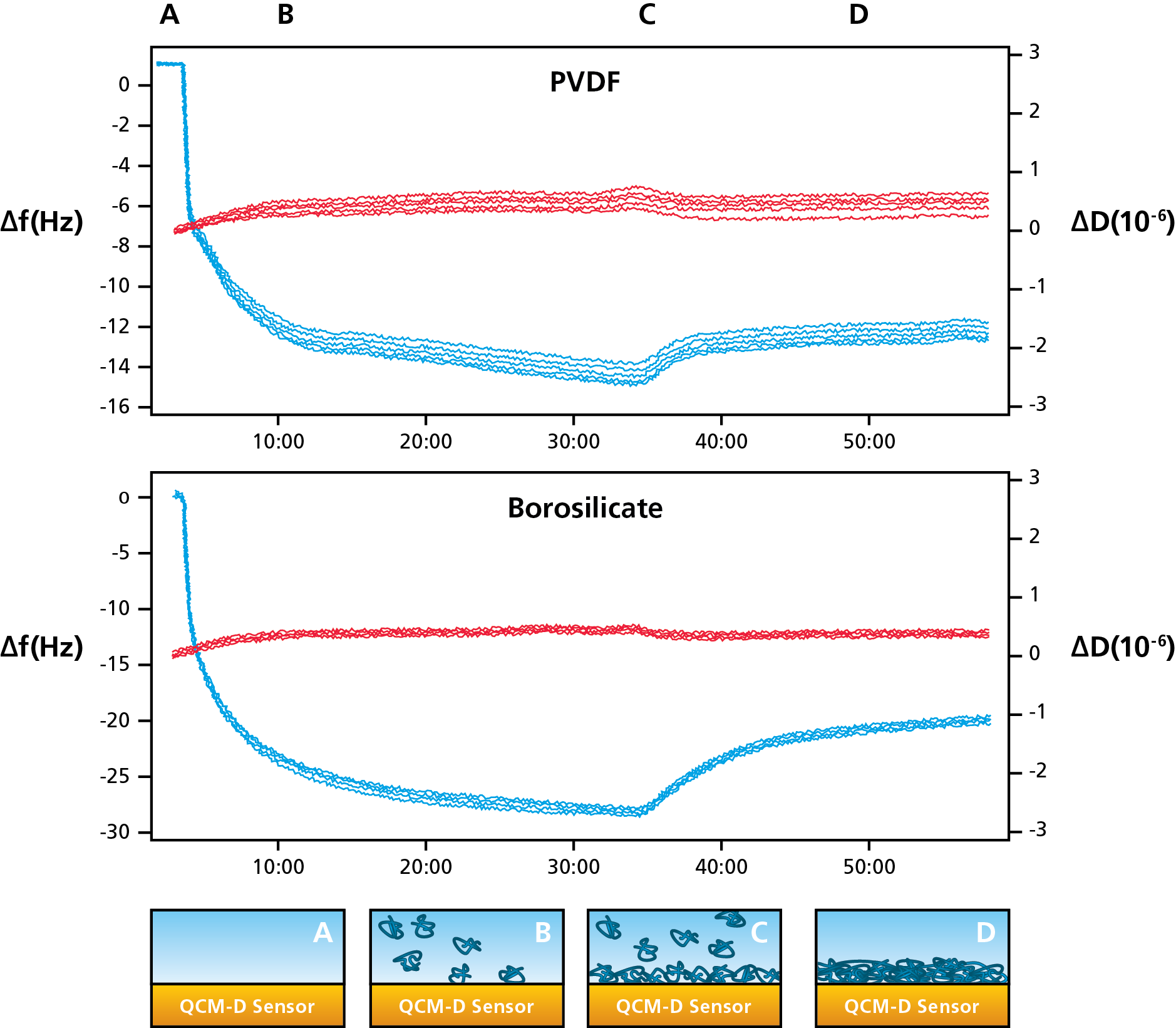
Figure 1. (Top) Protein adsorption on plastic (PVDF) and glass (borosilicate) measured with QCM-D. (Bottom) Schematic illustration of the protein adsorption process.
Monitoring the mass as a function of time, evaluating surface interaction processes is straightforward. It is also possible to compare behavior under different conditions by varying for example the concentration, temperature, pH and ionic strength.
In addition to the example shown here, other types of adsorption and desorption events that could be characterized by measuring the mass uptake and mass loss include, for example, surface interaction of surfactants, polymers and nanoparticles.
Download the overview to read more about what information you can obtain with QSense QCM-D.
Register for the webinar
Read the guidelines on how to decide which QCM instrument will be the most suitable for your needs
Read about how protein adsorption at various surface and solution conditions quickly can be measured
Read about what single-harmonic and multi-harmonic QCM-D means and what the difference is between these instruments.
Read about how QSense QCM-D analysis is used as a powerful tool to investigate protein-lipid nanoparticles binding affinity
Learn about the difference between the theoretical QCM sensitivity and the sensitivity which is relevant in a measurement situation.
Read about the piezoelectric effect and how piezoelectricity arises
Learn more about QSense 4th generation QCM-D platform which provides a sharper tool in the scientist toolbox and simplifies data interpretation.
Learn about how biointerfaces and biomolecular interactions can be studied using QSense® QCM-D and what information these measurements offer.
Read about Prof. Jackman's experience using QCM-D to study surfactant-interaction with model membranes
Sign upp for the webinar to learn more about how QCM-D is used to study biomaterial-induced activation of the immune system
