Surface free energy can be considered as the surface tension of a solid. The unit of surface free energy is mN/m which is equivalent to dynes/cm. Surface free energy, or SFE for short, arises from the molecular interactions at the air – solid interface.
Surface free energy is important in many application areas. It dictates how the solid behaves when put in contact with liquids. The surface free energy of a solid predicts how a material behaves when placed in a human body or how a coating formulation spreads on it.
Interactions between solid and liquid are important in many processes as they determine the adhesion between the two phases. Solid-liquid interactions are determined by the surface free energy of the solid and the surface tension of the liquid applied. When the surface free energy of the solid is high, it is usually easily wetted by any liquid, such as paints.
Surface free energy cannot be directly measured. Water contact angle measurement alone indicates the wetting of the solid, but the surface free energy is the quantitative measure of the intermolecular forces at the surface which is independent of the liquid used. By knowing the surface free energy of the solid, one can predict the behavior of any liquid on the surface.
Term surface free energy describes the excess energy that the surface has compared to the bulk of the material. At the bulk, the molecules have similar molecules on their sides and are pulled equally to all directions.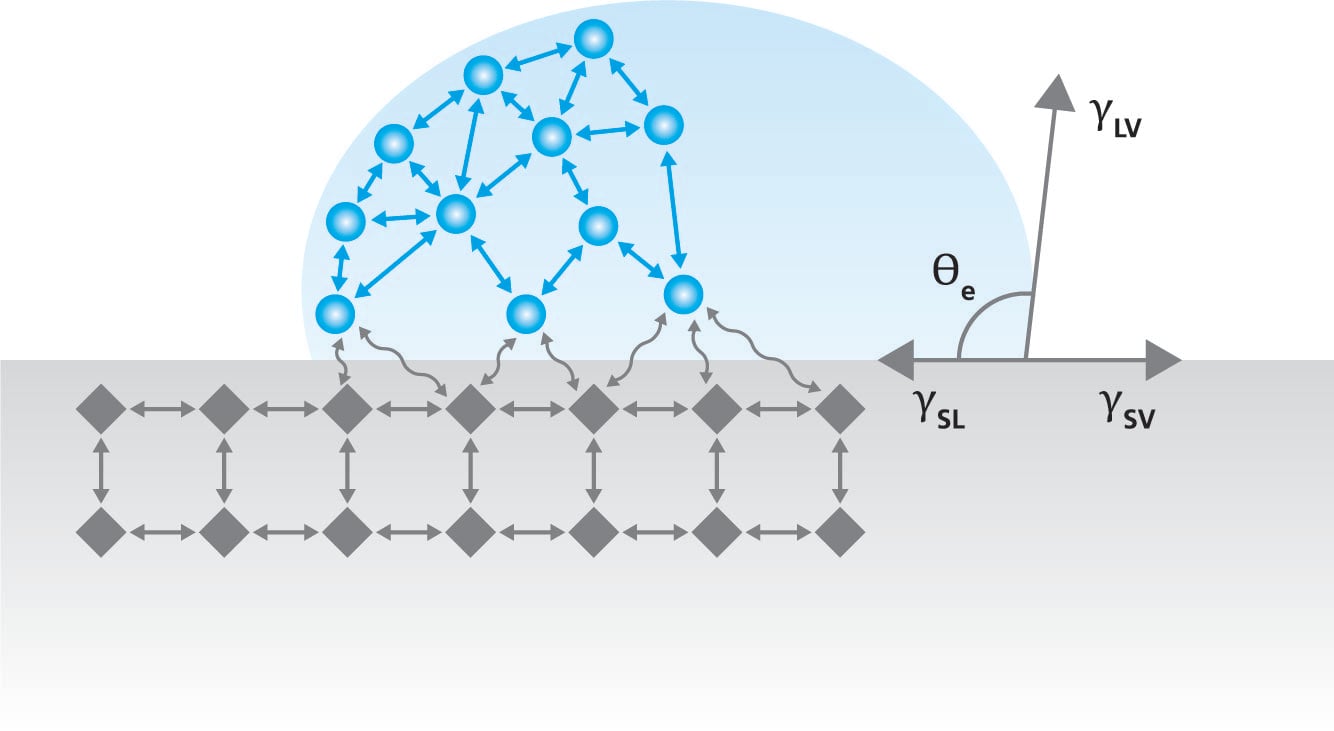
This causes a zero-net force on the molecule. However, at the air – solid interface, the molecules have similar neighboring molecules only on their side and below. There is very little interaction with the molecules in air which causes excess energy at the solid interface. The magnitude of the surface free energy depends on the interaction between the molecules. In the case of metals, the surface free energy is high due to strong metallic bonds between the metal atoms. In polymers, the molecular forces are typically much weaker and thus the surface free energy of the polymers is low.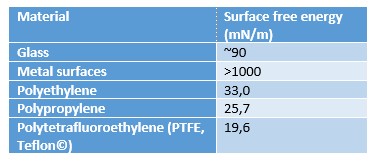
The most commonly used surface free energy theory, OWRK, divides the surface into two components: polar and dispersive. The dispersive interactions include van der Waals forces such as London dispersive force, Debye inductive force, and Keesom orientational force. Polar interactions are hydrogen bonding and dipole-dipole interactions. The polar interactions can be further divided into acid and base components.
The surface free energy of the solid can be predicted by water contact angle measurement. If the water spreads on the solid surface, the surface free energy is high whereas if it beads up the SFE is low. On a clean glass surface, the water contact angles are typically quite low and whereas on polymer surfaces the water contact angles are high.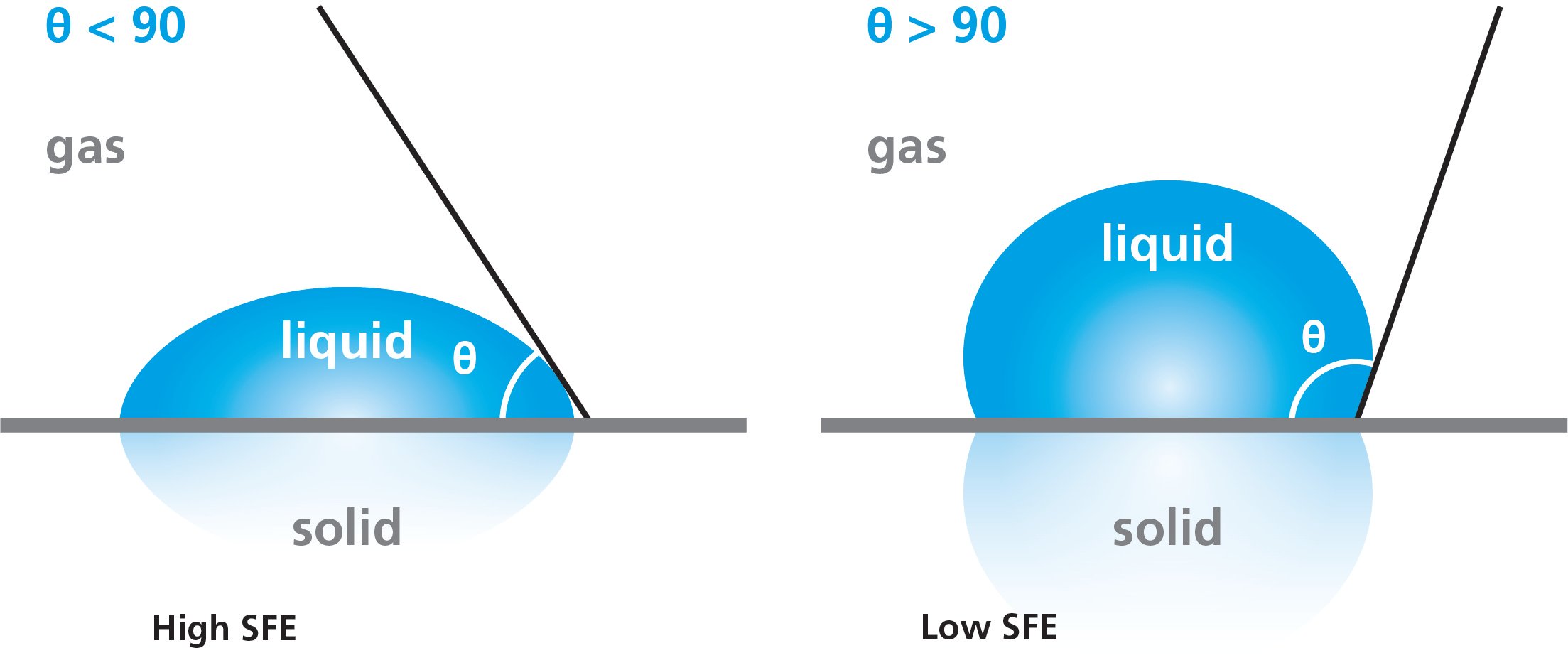
The water contact angle measurement is not able to give a numerical value for surface free energy. To calculate the surface free energy, measurements with at least two probe liquids are required. The surface free energy calculations are based on the modeling of the interfacial tension between the liquid and solid. There are several theories based on which the interfacial tension is modeled. The modern contact angle measurement software can typically give SFE value based on different theoretical models.
To read more about surface free energy measurements and theories behind, please download the overview below.
Editors note: This blog was originally published 27th of November 2018 and has since been updated for completeness.
OWRK is one of the most used surface free energy theories. It divides the interfacial interactions into two parts; polar and dispersive.
Surface free energy can be calculated through contact angle measurements. Automated instruments are available.
You might find it confusing when seeing all the different methods for surface free energy calculations. As surface free energy can give you more information on your surface compared to the contact angle measurements alone, it is time well spent to study the different theories more in detailed.
Calculate critical surface tension or surface free energy for a solid by testing against a series of liquids and measuring the contact angles.
